How Covid Vaccine Trial Works
A Phase II trial is larger and involves volunteers being given a. A second shot a few weeks later is needed to get the most protection the vaccine has to offer.
 Covid 19 Vaccine Trials 9 Things You Should Know Hackensack Meridian Health
Covid 19 Vaccine Trials 9 Things You Should Know Hackensack Meridian Health
Phase 1 testing marks the first time the vaccine is tested in a small group of adults usually between 20 to 80 people to evaluate its safety and measure the immune response it generates.
How covid vaccine trial works. Messenger RNA mRNA vaccine. All but one of the COVID-19 vaccines that are currently in Phase 3 clinical trials in the United States use two shots. To find out whether experimental Covid-19 vaccines are safe and effective researchers design clinical trials involving thousands of volunteers divided into groups that receive either.
The COVID-19 mRNA vaccines that BioNTech-Pfizer and Moderna developed are the first mRNA vaccines authorized for use in humans outside of clinical trials. 1158 AM CDT October 22 2020. Assessing whether the vaccine prevents transmission which is probably a prerequisite for.
Once a Phase I trial has established an optimal dose and determined the vaccine is safe a vaccine moves on to a Phase II trial. Based on evidence from clinical trials the Pfizer-BioNTech vaccine was 95 effective at preventing laboratory-confirmed COVID-19 illness in people without evidence of previous infection. A piece of coronavirus The Sars-CoV-2 virus is studded with proteins that it uses to enter.
Some of the volunteers will get an injection containing the vaccine candidate and others will get an injection of an inert placebo. The main types of COVID-19 vaccines currently available in the US. Following the news that the Pfizer BioNTech candidate appears to be 90 per cent effective in preventing disease we take a closer look at how vaccine trials work.
Phase 2a studies aim to determine the most effective dose and expand the safety experience with the vaccine. Throughout all phases the trial is a double-blind study which means neither the researchers nor the patient know whether they are being given the COVID-19 vaccine or a placebo injection of. The vaccine produced strikingly high levels of antibodies in early clinical trials.
One vaccine in Phase 3 clinical trials only needs one shot. A clinical trial demonstrated that the vaccine has an efficacy rate of 95 percent in preventing Covid-19. Vaccine Development Coronavirus Variants Treatments Answers to Your Covid-19 Questions How the Covid-19 Vaccines Work.
How the COVID-19 vaccine trials work The race for a coronavirus vaccine has a fast-track contender the mRNA vaccine which has been developed in a matter of months. The race for a coronavirus vaccine has a fast-track contender the mRNA vaccine which has been developed in a matter of months. Throughout all phases the trial is a double-blind study which means neither the researchers nor the patient know whether they are being given the COVID-19 vaccine or a placebo injection of.
However the technology is not new. These trials recruit 10 to 100 healthy volunteers who receive different doses of vaccine. CDC will continue to provide updates as we learn more about how well the Pfizer-BioNTech vaccine works in real-world conditions.
Vaccine Trial How the COVID-19 vaccine trials work. Channel 2s Michael Seiden spoke with medical experts on how the vaccine trial process. The first shot starts building protection.
Clinical trials run by the state-owned company. This type of vaccine uses genetically engineered mRNA to give your cells instructions for how to make a harmless piece of the S protein found on the surface of the COVID-19 virus. Robin Wilhoit Jeremy Campbell Published.
The Maryland-based company Novavax has developed a protein-based coronavirus vaccine called NVX-CoV2373. ATLANTA Channel 2 Action News is getting more insight into COVID-19 studies happening in metro Atlanta. The trial was designed to test for symptomatic covid-19 and confirmed infection with the virus.
Robin Wilhoit Jeremy. Or in large-scale clinical trials include. Phase 1 trials are the first evaluations of a vaccine in humans.
Trials Underway for Necessary COVID-19 Vaccine for Teens By Terry McSweeney Published March 2 2021 Updated on March 3 2021 at 1209 pm NBC Universal Inc. The intention is to enroll at least 30000 volunteers per trial.
Pfizer Claims Coronavirus Vaccine Success Plans For Emergency Ok
 Covid 19 Vaccine Race Month 6 First Emergency Use Phase 3 Trials Absolutely Maybe
Covid 19 Vaccine Race Month 6 First Emergency Use Phase 3 Trials Absolutely Maybe
 Pfizer Vaccine Results Are Promising But Lack Of Data Very Concerning Experts Say
Pfizer Vaccine Results Are Promising But Lack Of Data Very Concerning Experts Say
 Covid 19 Vaccine Trials In Georgia Attract Healthcare Workers 11alive Com
Covid 19 Vaccine Trials In Georgia Attract Healthcare Workers 11alive Com
 Moderna S Clinical Trial Just Entered Phase Three Here S How Mrna Vaccines Work
Moderna S Clinical Trial Just Entered Phase Three Here S How Mrna Vaccines Work
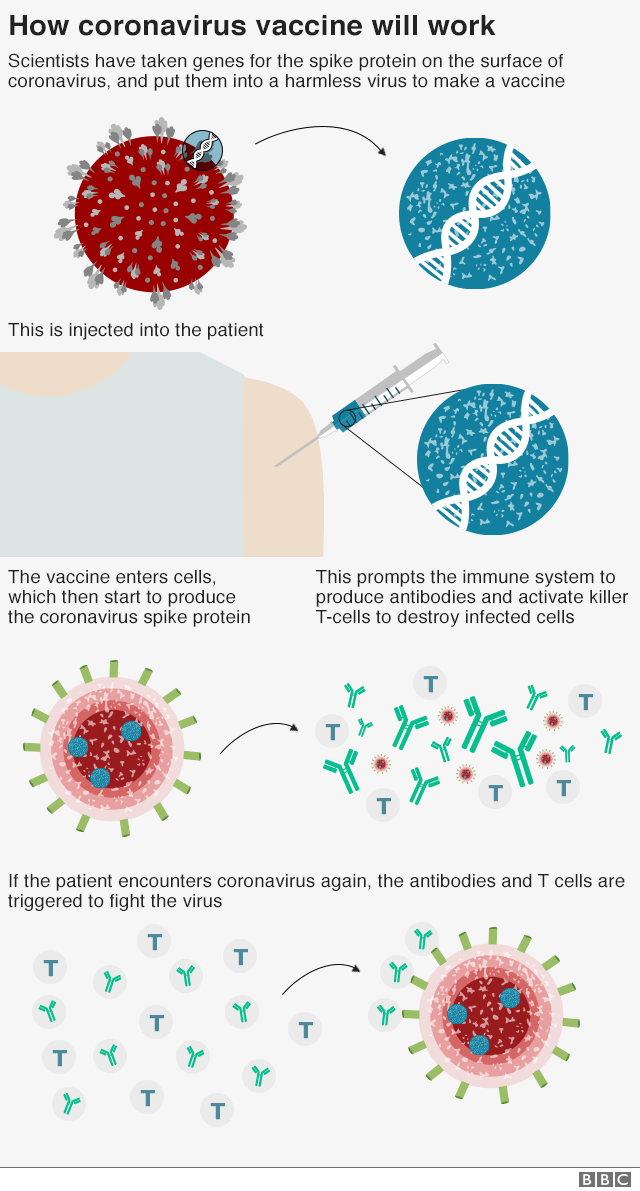 Coronavirus First Patients Injected In Uk Vaccine Trial Bbc News
Coronavirus First Patients Injected In Uk Vaccine Trial Bbc News
 A Covid 19 Vaccine Will Work Only If Trials Include Black Participants Experts Say
A Covid 19 Vaccine Will Work Only If Trials Include Black Participants Experts Say
 Astrazeneca Reveals Dosing Mistake In Coronavirus Vaccine Trials Euronews
Astrazeneca Reveals Dosing Mistake In Coronavirus Vaccine Trials Euronews
 Why A Coronavirus Vaccine Could Take Way Longer Than A Year
Why A Coronavirus Vaccine Could Take Way Longer Than A Year
 Oxford Covid Vaccine Works In All Ages Trials Suggest World News The Guardian
Oxford Covid Vaccine Works In All Ages Trials Suggest World News The Guardian
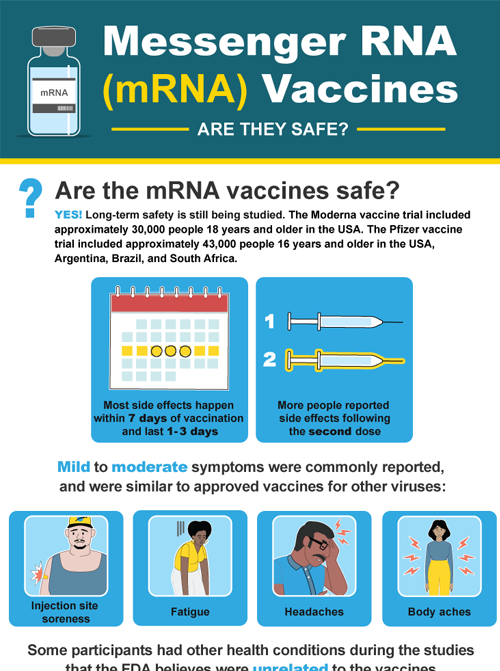 Science Of Vaccines And Monoclonal Antibodies Covid 19 Prevention Network
Science Of Vaccines And Monoclonal Antibodies Covid 19 Prevention Network
 The 5 Stages Of Covid 19 Vaccine Development What You Need To Know About How A Clinical Trial Works Johnson Johnson
The 5 Stages Of Covid 19 Vaccine Development What You Need To Know About How A Clinical Trial Works Johnson Johnson
 What Is Mrna How Pfizer And Moderna Tapped New Tech To Make Coronavirus Vaccines
What Is Mrna How Pfizer And Moderna Tapped New Tech To Make Coronavirus Vaccines
 Large Trials For The Oxford Covid 19 Vaccine Begin In The U S
Large Trials For The Oxford Covid 19 Vaccine Begin In The U S
South Africa Coronavirus Variant May Evade Vaccines Cause Reinfection
 How The Vaccine Works Faculty Of Medicine Imperial College London
How The Vaccine Works Faculty Of Medicine Imperial College London
 Coronavirus Vaccine Clinical Trial Starting Without Usual Animal Data Stat
Coronavirus Vaccine Clinical Trial Starting Without Usual Animal Data Stat
Coronavirus Vaccine First Dose Administered In Clinical Trial
Post a Comment for "How Covid Vaccine Trial Works"